On August 25, CMS released an Interim Final Rule that outlined five major provisions applicable for the duration of the public health emergency.
1. New provisions outline CMS’ intention to re-enforce compliance with LTC facility requirements for reporting information related to COVID-19 by implementing an automated process within ASPEN (Automated Survey Process Environment) to determine provider compliance with reporting requirements. This increase in CMS enforcement requires LTC facilities to comply with reporting new LTC COVID-19 data or risk a $1,000 for first incidence of non-compliance and an additional $500 for each subsequent occurrence up to a maximum of $6,500. (Note: Non-compliance will be categorized as an “F” deficiency of widespread scope and severity level and “no actual harm with potential for more than minimal harm that is not immediate jeopardy” and receive a F884 tag.)
2. CMS outlined expectations for LTC facility resident and staff testing to be conducted according to CDC guidelines found here: https://www.cdc.gov/coronavirus/2019-ncov/hcp/nursing-homes-testing.html. Revised LTC infection control regulations (§ 483.80) requires handwritten standards, policies and procedures for infection control including when and to whom incidents of communicable diseases or infections should be reported. Moreover, facilities are required to:
- Maintain documented detail for every test (including when conducted and results);
- Address those residents and staff unwilling to consent to testing via a well-defined plan;
- Helpful Tip: Give some thought to - how to delicately address resistance and keep the residents and staff safe. The rule states providers must have a well-defined plan. Provide the title of specific individuals who will be responsible for these difficult discussions and document each approach.
- Ensure minimum required staffing levels are maintained at all times (Note: Staff includes any individual employed by the facility, providing services under arrangement to the facility or volunteers);
- Coordinate with state and local health departments and/or CLIA certified labs RE: testing availability, access, processing, and receiving results as well as address any issues/considerations via facility infection prevention and control plan;
- Continue to report COVID-19 information to the CDC’s National Healthcare Safety Network (NHSN) in addition to reporting in accordance with CLIA requirements if testing under a CLIA certificate of waiver. Note: Facilities testing under a CLIA certificate of waiver are subject to regulations requiring labs to report data for all completed tests;
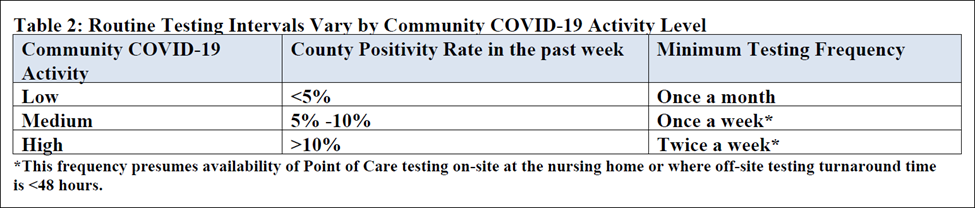
- Comply with revised COVID-19 Focused Survey for Nursing Homes outlined here and here https://www.cms.gov/files/document/qso-20-38-nh.pdf https://www.cms.gov/files/document/qso-20-37-clianh.pdf that define testing parameters including details such as testing frequency, diagnosis, symptom presentations, county positivity rate and response time for results. Specifically, a new COVID-19 positive resident or staff member requires all residents and staff that tested negative on initial test should to be tested and retested every 3-7 days until no new cases are identified for 14 days.
- Conduct routine testing – based on number of positive COVID-19 cases and according to the county’s positivity rate from prior week https://data.cms.gov/stories/s/COVID-19-Nursing-Home-Data/bkwz-xpvg
3. In March 2020, Extraordinary Circumstances Exceptions (ECE) was granted for four value-based purchasing programs and the performance period for the FY 2022 SNF VBP Program. CMS shared this was due to stakeholder concerns related to geographic differences in COVID-19 and the need to reduce data collection and reporting burden to let providers focus resources on patient care during the PHE. The Interim Final Rule specifies the new performance period for FY 2022 SNF VBP program will be April 1, 2019 to December 31, 2019 and July 1, 2020 through September 30, 2020 (eliminating Q1 and Q2 of 2020). In addition, CMS may propose to not score facilities in the SNF VBP program based on limited data or make associated payment adjustments for the affected payment year.
4. The Interim Final Rule states that one COVID-19 test plus one other applicable related test may be completed without physician orders. This revised rule -- to address concerns that patients may not have access to adequate medical care and quarantine when necessary – specifies that multiple tests without an order prior to publication of this rule would not count, citing that labs are a significant fraud and abuse risk.
5. In order to be reimbursed under Medicare Part B, pharmacists and auxiliary practitioners who order lab tests (in accordance with state practice and scope of practice) still need to be functioning in an incident-to-arrangement with a physician or non-physician practitioner. For the duration of PHE, the order of the physician or other practitioner is not required for one (otherwise covered) diagnostic test for COVID-19, but is required for subsequent tests.
Please note: although there is a comment period in effect until the 60th day following publication of the Interim Final Rule in the Federal Register, the final rule establishes new requirements to be enforced vs. a Proposed Rule with comment period.
HealthPRO Heritage is pleased to offer summaries and clarify regulatory updates for convenience and in support our partners and industry peers. Please share this update among your colleagues and/or on LinkedIn. Please note: our compliance and clinical strategy experts are available to respond to questions/provide commentary on all related topics and can be contacted here.